The groundwork for building a disease-specific pediatric cancer data commons was first laid by the INRG Data Commons, which was launched in 2014 based on work dating back to 2004, and continued with the development of the INSTRuCT Data Commons in 2017-2018. Based on these successes, we have significantly streamlined the process of creating a new disease commons and have been able to rapidly establish work with additional disease areas, with more yet to come.
Our Progress
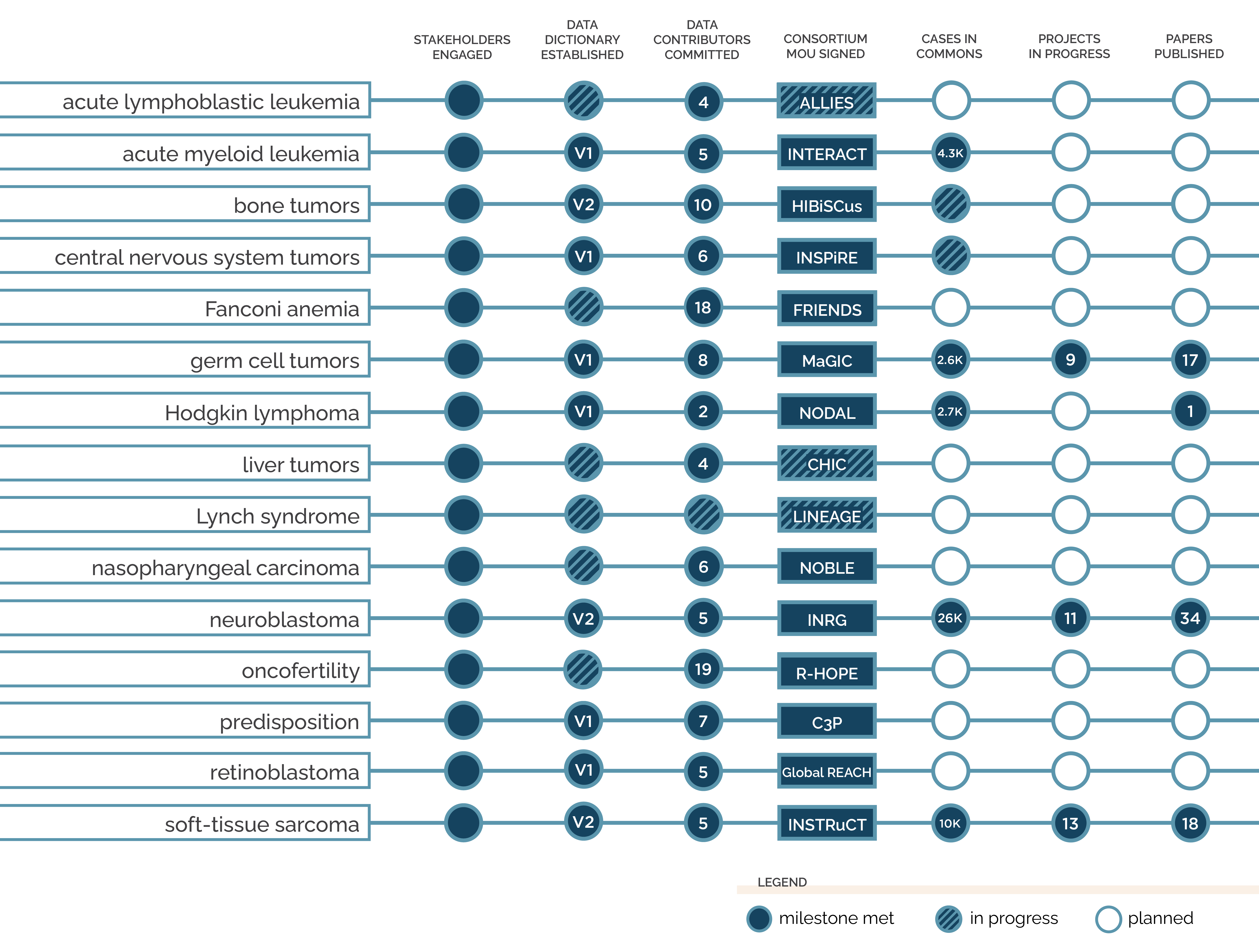
This tracker is updated quarterly. Last updated November 2025.
What do these milestones mean?
Stakeholders Engaged
In some cases, pre-established consortia have approached the PCDC to create a disease-specific commons. In other cases, we work closely with leaders in a specific pediatric cancer type to identify interested cooperative research groups who are willing to form a consortium to guide development of the commons and steer future progress.
Data Dictionary Established
The PCDC data standards team joins a work group of researchers and clinicians with disease-specific knowledge to develop the data dictionary, by which all data in the commons will be standardized. This data dictionary is created using case report forms from existing studies, and is ballotted and agreed upon by disease group experts and statisticians. The work group also establishes a change control process for future updates.
The number in the progress tracker represents the version of the data dictionary currently in use.
Data Contributors Committed
The initial data in the commons is contributed by the participating cooperative groups, which have already collected clinical trials data from their part of the world in a centralized way. Each cooperative group contributing data to the commons signs a Data Contributor Agreement (DCA), a legal contract between the contributing institution and the University of Chicago.
The number in the progress tracker represents the number of committed data contributors.
Consortium MOU Signed
A Memorandum of Understanding (MOU) is agreed upon and signed by all committed parties, and the consortium is officially formed. An executive committee is established, composed of representatives from each cooperative group and experts from key disciplines. This committee is responsible for strategic planning as well as reviewing and approving new members, contributions of data to the commons, and requests from researchers for access to data. Operating procedures are established, and work groups are created to drive specific areas of work such as governance and data dictionary development.
Cases in Commons
Once the consortium and data dictionary have been established and DCAs have been signed, data can be ingested into the commons. Cooperative groups within the consortium provide mapped and harmonized data from their closed clinical trials. The PCDC team performs quality control checks and integrates each set of contributed data into the commons where it can be combined and cross-referenced with other data. Our technology team also sets up a cohort discovery tool to make it easier for researchers to explore the commons.
The number in the progress tracker represents the number of cases currently available in the commons.
Projects in Progress
To receive access to data in the commons, investigators submit a project proposal to the consortium executive committee. If the proposal is approved, the University of Chicago and the requesting institution sign a Data Use Agreement (DUA), a legal document outlining how the data may be used. The relevant dataset is then securely provided to the researcher. Consortium members also collaborate in work groups to write consensus papers.
The number in the progress tracker represents the number of projects currently in progress, including both data-driven and consensus papers.
Papers Published
Finally, investigators use data from the commons to conduct analyses and publish or present their findings. These discoveries, made from data that might otherwise have sat siloed and unused, can now inform future research and clinical practices such as risk stratification, ultimately leading to better outcomes for children with cancer. PCDC consortium members also come together in interdisciplinary groups to write expert consensus papers that can guide future research and care.
The number in the progress tracker represents the number of published manuscripts resulting from data obtained from the commons, as well as consensus papers produced by PCDC consortia.